This is the comparison of the COVID-19 vaccines that are considered for their next application in Latin America.
Entrepreneur’s New Year’s Guide
Let the business resources in our guide inspire you and help you achieve your goals in 2021.
February 3, 2021 9 min read
EDITOR’S NOTE: THIS ARTICLE WILL BE CONSTANTLY UPDATED.
Vaccination against the SARSCov2 coronavirus that causes the COVID-19 pandemic in the world will be a central issue for all the countries of the world in the coming years while the long-awaited “herd immunity” is achieved, that is, when the virus already it cannot be spread because most people will be protected against it.
There are at least 12 vaccines on the market already in application or in the last phases of their clinical trials, waiting to be endorsed by the health authorities of the United States, the European Union or that of each country for emergency use.
Do vaccines cure COVID-19?
The simplest answer: No.
The objective of vaccines is to reduce the virulence of the pathogen, that is, to help prevent fatal cases and the impact of vulnerable groups in the population.
Do vaccines have microchips?
The World Health Organization’s Immunization Program emphasizes that no current vaccine can modify people’s DNA.
It should be noted that they neither have chips nor are they made from human fetuses. They also do not cause autism or cause infertility.
What vaccines are already licensed?
So far, the WHO has only globally licensed Pfizer’s vaccine. Moderna’s is expected to get its approval in February, although it has already been endorsed by the US Food and Drug Administration (FDA). AstraZeneca could also be licensed for worldwide use in the coming weeks.
Johnson & Johnson, Sinopharm, Sinovac and CanSino vaccines could be approved by the WHO between February and June.
Sputnik V, -which has the authorization for emergency use in Mexico, Russia, Argentina, Algeria, Belarus, United Arab Emirates, Hungary, Palestine, Paraguay, Pakistan, Serbia, Turkmenistan and Venezuela-, still has no validation date for part of the world body.
Comparison of COVID-19 vaccines
This is the comparison between the 9 best-known vaccines so far.
1. Pfizer / BioNTech

Image: Depositphotos.com
- Estimated efficiency: 95%
- Country of origin: United States and Germany
- Phase: Already in application.
- Price: $ 19.5 per injection .
- Method: They are based on a technology that had been studied for 20 years for its use in the treatment of various types of cancer. It involves the use of messenger RNA, which in very simple terms, teaches the cells of the human body to produce a protein that makes the immune system react in accordance with SARSCov2, the virus responsible for COVID-19.
- Application: Two injections at least 28 days apart.
- Pros: Proven very high efficacy to prevent symptomatic infections.
- Cons: It must be stored in a cold of at least -70 ° C. This raises its price.
2. Modern

Image: Depositphotos.com
- Estimated efficiency: 95%
- Country of origin: United States
- Phase: Already in application.
- Price: $ 37 per injection .
- Method: It is based on a technology that had been studied for 20 years for its use in the treatment of various types of cancer. It involves the use of messenger RNA, which in very simple terms, teaches the cells of the human body to produce a protein that makes the immune system react in accordance with SARSCov2, the virus responsible for COVID-19.
- Application: Two injections at least 28 days apart.
- Pros: Proven very high efficacy to prevent symptomatic infections.
- Cons: It can last 30 days at a temperature of up to 8 ° C , but should freeze to -20 ° C if stored for more days. This raises its price.
3. Sputnik V
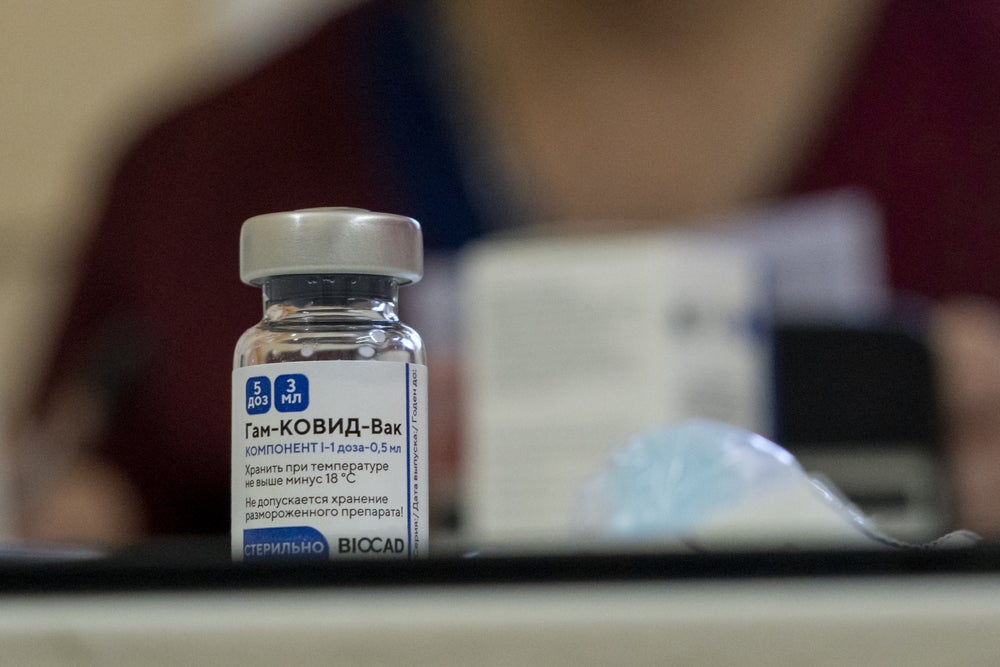
Image: Depositphotos.com
- Estimated efficacy: 91% according to The Lancet .
- Country of origin: Russia
- Phase: Already in application in several countries. It has just been endorsed in Mexico.
- Price: $ 10 per injection .
- Method: Use two different types of adenoviral vectors, viruses responsible for the common human cold.
- Application: Two injections 3 weeks apart.
- Pros: Proven high efficacy to prevent symptomatic infections. More accessible price.
- Cons: They are much easier and cheaper to produce and transport in a range of 2ºC and 8ºC .
4. AstraZeneca
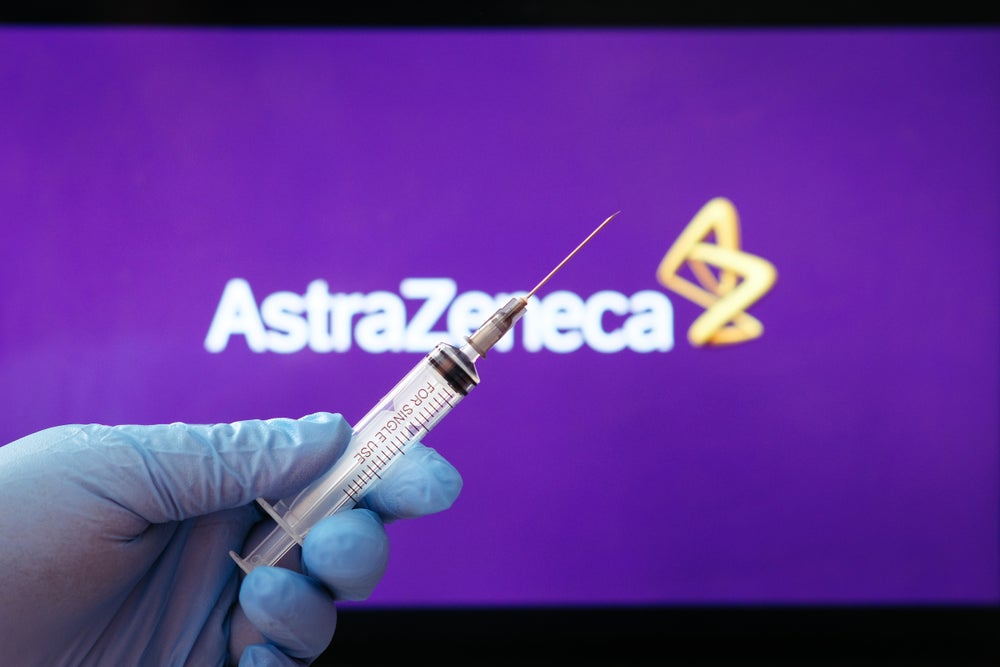
Image: Depositphotos.com
- Estimated efficiency: 70%
- Country of origin: UK and Sweden
- Phase: Already in application.
- Price: $ 10 per injection .
- Method: Uses a genetically altered virus with a coronavirus protein to activate the body’s immune response.
- Application: Two doses 4 weeks apart.
- Pros: They are much easier and cheaper to produce and transport as they can survive in standard refrigerators for up to six months.
- Cons: They are much easier and cheaper to produce and transport.
5. CanSino
Image: Depositphotos.com
- Estimated efficiency: About 90%.
- Country of origin: China
- Phase: III of clinical trial in countries such as Pakistan, Russia, Mexico and Chile. The Chinese Army approved its use in June . The president of Mexico, Andrés Manuel López Obrador announced that, if approved, – he would be vaccinated with it at the end of February.
- Price: It would be fixed in these weeks .
- Method: Use the traditional technique of inoculation with parts of the virus that have been inactivated with chemicals.
- Application: A single injection.
- Pros: They are much easier and cheaper to produce and transport as they can survive in standard refrigerators for up to six months.
- Cons: They are much easier and cheaper to produce and transport.
6. Novavax
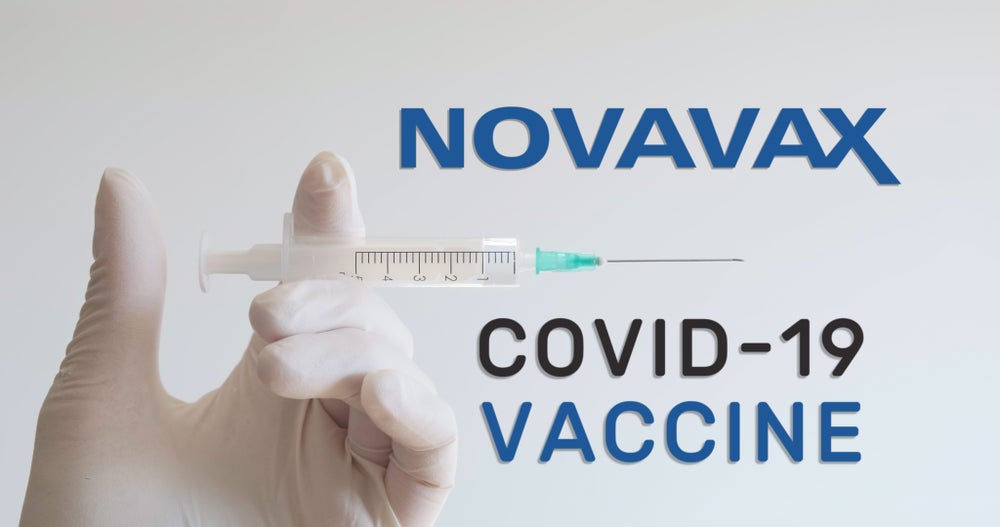
Image: Depositphotos.com
- Estimated efficacy: 95.6% against the original coronavirus , 85.6% against the UK strain.
- Country of origin: United States.
- Phase: III clinical trial
- Price: Not set.
- Method: Uses a genetically altered virus with a coronavirus protein to activate the body’s immune response.
- Application: Two doses at least 2 weeks apart.
- Pros: Proven high efficacy to prevent symptomatic infections. More accessible price.
- Cons: They are much easier and cheaper to produce and transport.
7. Sinopharm
Image: Depositphotos.com
- Estimated efficacy: There are no official data on its efficacy.
- Country of origin: China
- Phase: III clinical trial
- Price: Estimated at $ 145 .
- Method: Use the traditional technique of inoculation with parts of the virus that have been inactivated with chemicals.
- Application: Two doses at least 3 weeks apart.
- Pros: More affordable price; not the transgenic therapy that makes some people uncomfortable.
- Cons: They are much easier and cheaper to produce and transport.
8. Johnson & Johnson
Image: Depositphotos.com
- Estimated efficacy: 66 to 72% efficacy in the global trial .
- Country of origin: United States
- Phase: III of a clinical trial with volunteers from the United States, Argentina, Brazil, Chile, Colombia, Mexico, Peru and South Africa.
- Price: Not set.
- Method: Uses a genetically altered virus with a coronavirus protein to activate the body’s immune response.
- Application: A single injection.
- Pros: They are much easier and cheaper to produce and transport as they can survive in standard refrigerators for up to six months.
- Cons: Its effectiveness is lower than other proposals on the market.
9. Sinovac
Image: Depositphotos.com
- Estimated efficacy: 78% of mild cases.
- Country of origin: China
- Phase: Already in application in China in vulnerable groups and in countries such as Turkey, Singapore and the Philippines. His arrival is expected soon to Chile and Brazil.
- Price: $ 60.
- Method: Use the traditional technique of inoculation with parts of the virus that have been inactivated with chemicals.
- Application: Two injections 15 days apart .
- Pros: They are much easier and cheaper to produce and transport as they can survive in standard refrigerators for up to six months.
- Cons: They are much easier and cheaper to produce and transport.





